Bribing a scientist to find a beer's ABV
Determining the alcohol content of beer can be difficult to get right. There are generally two ways to do it, measuring specific gravity with a hydrometer or by measuring the index of refraction with a refractometer.
We use the refractometer method because it is quick, only requires a drop of beer, and lets us track the progress of a fermentation with relative ease.
The downside to both of these techniques is that they are prone to error unless you put in the effort to correct for the fact that beer is not just a solution of alcohol and water.
Generally this error is small and we're fine with being off by a bit on our calculations.
That was until we brewed the Best Man Barleywine. Since this was a wedding gift for a friend, we wanted to put in the effort to figure out the exact ABV of what we were giving him.
Like most things we do, we wanted to get a little extreme with it.
A little research showed that a Nuclear magnetic resonance spectrometer could be used to count the number of atoms in our beer and get a true ABV.

A NMR Spectrometer.
It turns out these machines are expensive. Luckily UW-Madison has a few, so we needed to find a chemist we could bribe with a 6-pack of our homebrew.
Dr. Charles Fry turned out to be more than willing to help and within a few minutes we had our measurements.
At the end of the day we measured two samples, one that was aged in the barrel and one that was not. Our barrel aged barley wine was exactly 12.4% and the pre-barrel sample was 11.7%.1
Dr. Fry was kind enough to walk us through the math. His explanation of the numbers follows:
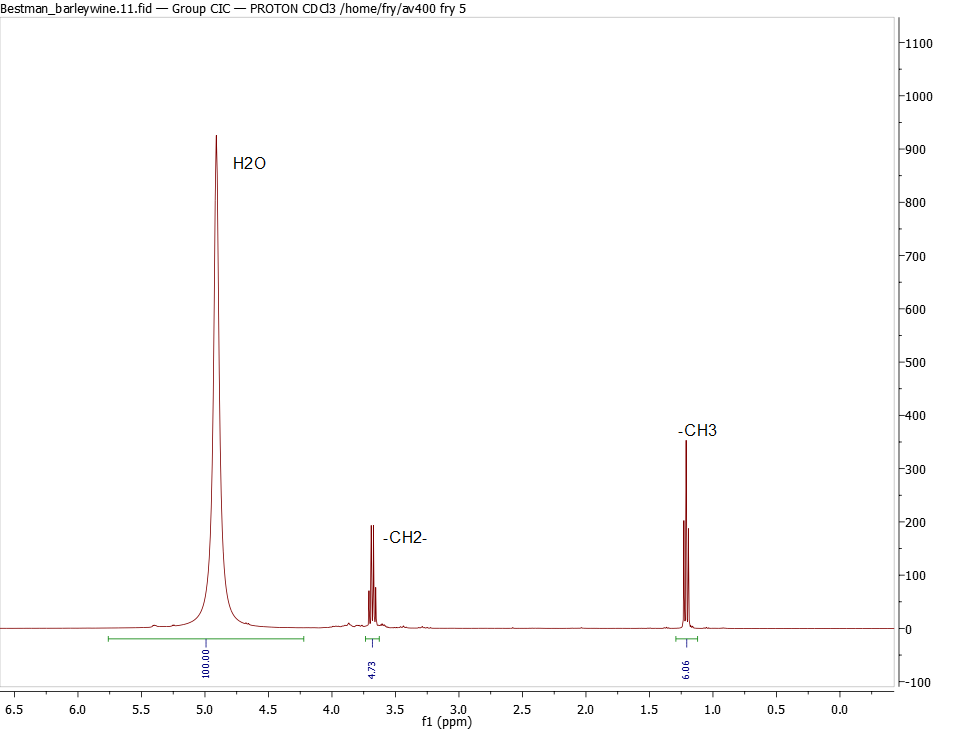
So let's take the spectrum above. The methyl peak -CH3 is the cleanest for measuring the ethanol content, and is 6% the size of the H2O peak. Two protons on water, three on the -CH3, so we take 6.06% * 2/3 = 4.04%. This is a measure of number of molecules: for every 100 water molecules there are 4.04 ethanol molecules. And I'm still not quite there. The -OH in ethanol is almost certainly sitting on the water peak. So we actually have only 96 H2O for each 4.04 ethanol molecules.
But we have to correct each measure by the molecular weight:
H2O: 96 molecules * 18 grams/mol = 1728 grams relative to ethanol: 4.04 molecules * 46 grams/mol = 185.8 grams
So the weight percent = 185.8 / (1728+185.8) = 9.7% by weight
2nd correction is to correct for specific gravity: 9.7% * 1 / 0.78 = 12.4% by vol alcohol for barleywine
The pre-barrel gave 5.68% initially, and that ends up with 11.7% by vol ethanol.
So there you have it, a third way to find out the ABV of your beer. All you need to do is bribe a chemist. Lets be honest here, who doesn't want to bribe a scientist?
Special thanks to Dr. Charles Fry PhD and the University of Wisconsin-Madison Department of Chemistry.
This means that a fresh 10 gal whiskey barrel will add ~1% ABV to what ever you are aging. ↩